
Radon 220
Measuring the half-life of radon 220 is a standard college physics experiment. I have
found that it is possible to replicate it at home using improvised equipment.
I recommend obtaining a copy of the
periodic table, as it will make the following descriptions easier to follow.
Radon is a naturally occurring radioactive gas. It can seep into the home from below
ground and pose a health risk. There are many different isotopes of radon. The type which
seeps from the ground is radon 222 which has a half-life of 3.8 days and decays by emitting
an alpha particle.
The half-life of a radioactive material is the time taken for half of the nuclei to
disintegrate. Different isotopes of the same element have the same number of protons
in the nucleus, but a different number of neutrons. The total number of protons and
neutrons in the nucleus is called the mass number 'A' and is the number given after the
name of the element. The number of protons only is called the atomic number 'Z', which
gives the position of the element in the periodic table. Different isotopes of the
same element have different half lives and sometimes different modes of decay, but all
have the same chemical properties. They differ by mass number 'A', but not by atomic
number 'Z'.
The usual classroom source of radon is thorium. One of the decay products of thorium is
radon 220. Because it is a gas, it can easily be separated from the thorium.
Primordial thorium (Th) has A=232 and Z=90. I will use the notation 90Th232. It
initially decays by emitting an alpha (α) particle. An α particle is the
same as a helium nucleus. It is made up of two protons and two neutrons and therefore
has an electronic charge of +3.2x10-19C. Using the same notation, an alpha
particle is 2He4. When 90Th232 emits a 2He4 it loses two protons and two neutrons,
becoming 88Ra228 (radium 228).
The notation for this is 90Th232 => 88Ra228 + α.
Ra228 decays by emitting a beta particle. A beta particle (β) is a high velocity
electron and therefore has a charge of -1.6x10-19C. The strange thing is that
this electron comes from the nucleus and as a result, one of the neutrons becomes a proton.
The mass number, A, stays the same, but the atomic number, Z, increases by one. The radium
228 then becomes actinium 228.
The notation for this is 88Ra228 => 89Ac228 + β
The sequence continues:
89Ac228 => 90Th228 + β
90Th228 => 88Ra224 + α
88Ra224 => 86Rn220 + α
We now have radon 220, which is a gas with a half-life of 55.6 seconds and decays by
alpha emission.
86Rn220 => 84Po216 + α
84Po216 => 82Pb212 + α
Fortunately, polonium 216 and lead 212 have very different half lives to radon 220 and so
will not significantly affect the measurement. Polonium 216 has a half-life of 0.15 seconds
and so will just double the number of counts recorded. Lead 212 has a half-life of 10.6 hours
and so will not be significant.
 An easily obtainable source of thorium is old but unused gas light mantles like the
one on the left. There is not much thorium in a mantle and so at least four
are required to measure the half-life of radon.
An easily obtainable source of thorium is old but unused gas light mantles like the
one on the left. There is not much thorium in a mantle and so at least four
are required to measure the half-life of radon.
Thorium mantles are reasonably safe to handle as long as sensible precautions are taken
to avoid contamination. It is probably much safer to use them for this experiment than
for their intended use. Used mantles are more hazardous than new ones as they become
brittle and powdery with use and can then spread radioactive particles which could be
inhaled.
Interestingly, the instruction sheet, packed with the mantle, is also radioactive as a
result of the radon decay products.
 I placed four mantles in a plastic bottle. I drilled a hole in the lid and attached a
short length of plastic tube. The lid and the tube were then sealed with tape and
Blu-tack.
I placed four mantles in a plastic bottle. I drilled a hole in the lid and attached a
short length of plastic tube. The lid and the tube were then sealed with tape and
Blu-tack.
The bottle was then left for an hour to allow the radon to accumulate.
To detect the alpha particles, I used a Nuclear Enterprises PCM5/1 contamination monitor
with a AP3/4A probe. I obtained this instrument on eBay. The AP3/4A probe is only sensitive
to alpha particles, so there is practically no background count.
Unfortunately the PCM5/1 does not have an output to connect to a counter, so I had to add
one. To do the counting I used a Philips PM6666 counter/timer.
 I taped a freezer bag over the alpha probe, to hold the radon during counting.
I taped a freezer bag over the alpha probe, to hold the radon during counting.
To transfer the radon to the bag, the Blu-tack was removed and the end of the tube inserted
into the corner of the bag. The bottle was squeezed flat and then the tube was removed from
the bag and the corner taped over.
A timer was then started, simultaneously with the gate being opened on the counter. The count
was then recorded every ten seconds for five minutes.
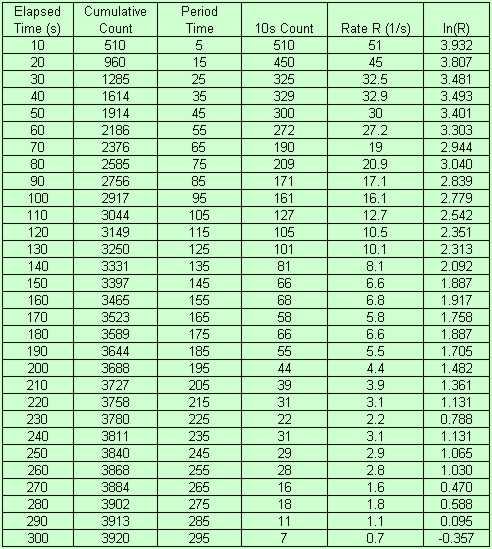 The results are shown in the table on the left.
The results are shown in the table on the left.
The first column is the time at which each count was recorded. The next column is what the
count had reached by that time. The Period Time is the center of each count window. 10s Count
gives the number of counts since the last count was recorded. Rate R is the count rate at that
Period Time and was obtained by dividing the 10s Count by 10. ln(R) is the natural log of the
count rate 'R'.
R = R0 e-λt
where t is the elpsed time in seconds (Period Time), R0 is the initial count rate
at t=0 and λ is the decay constant.
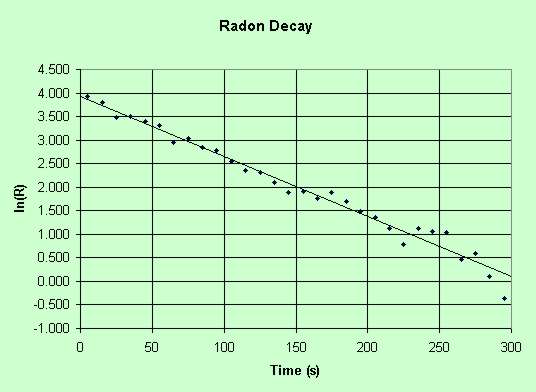
When the natural log of the count rate is plotted against time, the slope of the graph
gives the decay constant -λ. The half-life is given by -0.6931/Gradient. From the
graph below, λ = 3.83/300 = 0.01277. The half-life works out as 0.6931/0.01277 =
54.3 seconds. This is 2.3% lower than the published figure of 55.6 seconds.
The statistics could be improved by using more mantles.
 An easily obtainable source of thorium is old but unused gas light mantles like the
one on the left. There is not much thorium in a mantle and so at least four
are required to measure the half-life of radon.
An easily obtainable source of thorium is old but unused gas light mantles like the
one on the left. There is not much thorium in a mantle and so at least four
are required to measure the half-life of radon.
 I placed four mantles in a plastic bottle. I drilled a hole in the lid and attached a
short length of plastic tube. The lid and the tube were then sealed with tape and
Blu-tack.
I placed four mantles in a plastic bottle. I drilled a hole in the lid and attached a
short length of plastic tube. The lid and the tube were then sealed with tape and
Blu-tack. I taped a freezer bag over the alpha probe, to hold the radon during counting.
I taped a freezer bag over the alpha probe, to hold the radon during counting. The results are shown in the table on the left.
The results are shown in the table on the left.